The pKb of ammonia is 4.75 at 25°C. Answer. As shown in part (b) in Figure 17.4.3, the titration curve for NH3, a weak base, is the reverse of the titration curve for acetic acid. In particular, the pH at the equivalence point in the titration of a weak base is less than 7.00 because the titration produces an acid.. Adding the titrating solution to the titrand’s solution. Using the red stopper at the bottom of the buret, slowly add the titrating solution to the titrand’s solution one drop at a time. It is important to only let the titrating solution be added one drop at a time because the titration reaction can be very sensitive.
Titrations

Screening results of various titrations Download Table

PPT Calculating concentrations PowerPoint Presentation, free download ID3199214

Titration 2 Class 12 CBSE Practical All Study Guide at one Place!

Chemistry Techniques Titration Compound Interest
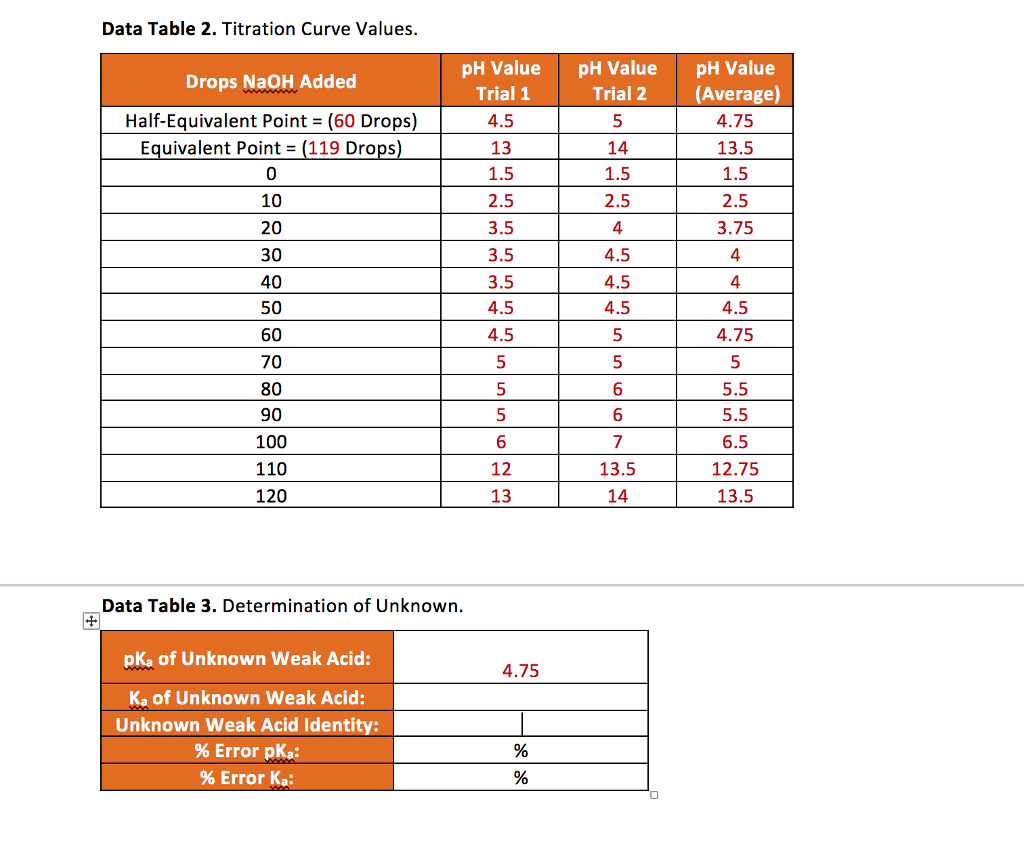
Solved Data Table 2. Titration Curve values. Drops NaOH
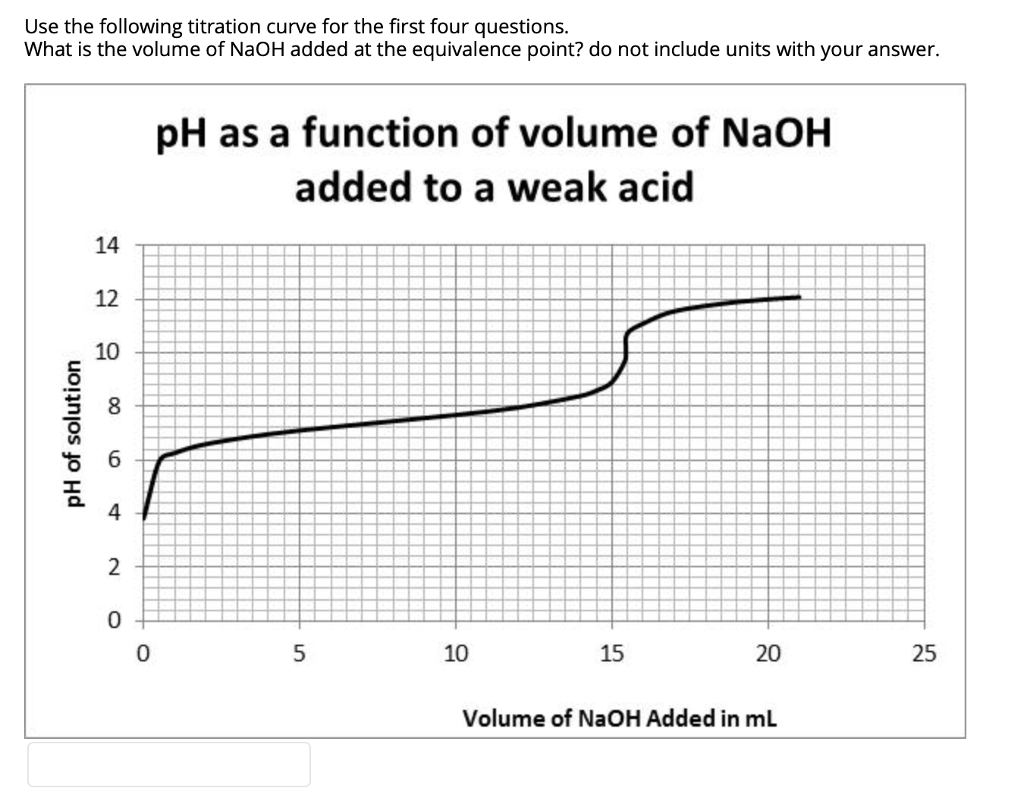
Solved Use the following titration curve for the first four

Titration Experiments In Chemistry The Chemistry Blog

BTEC Medical science titration results table

3.5 tabulating of results and calculating average titre YouTube
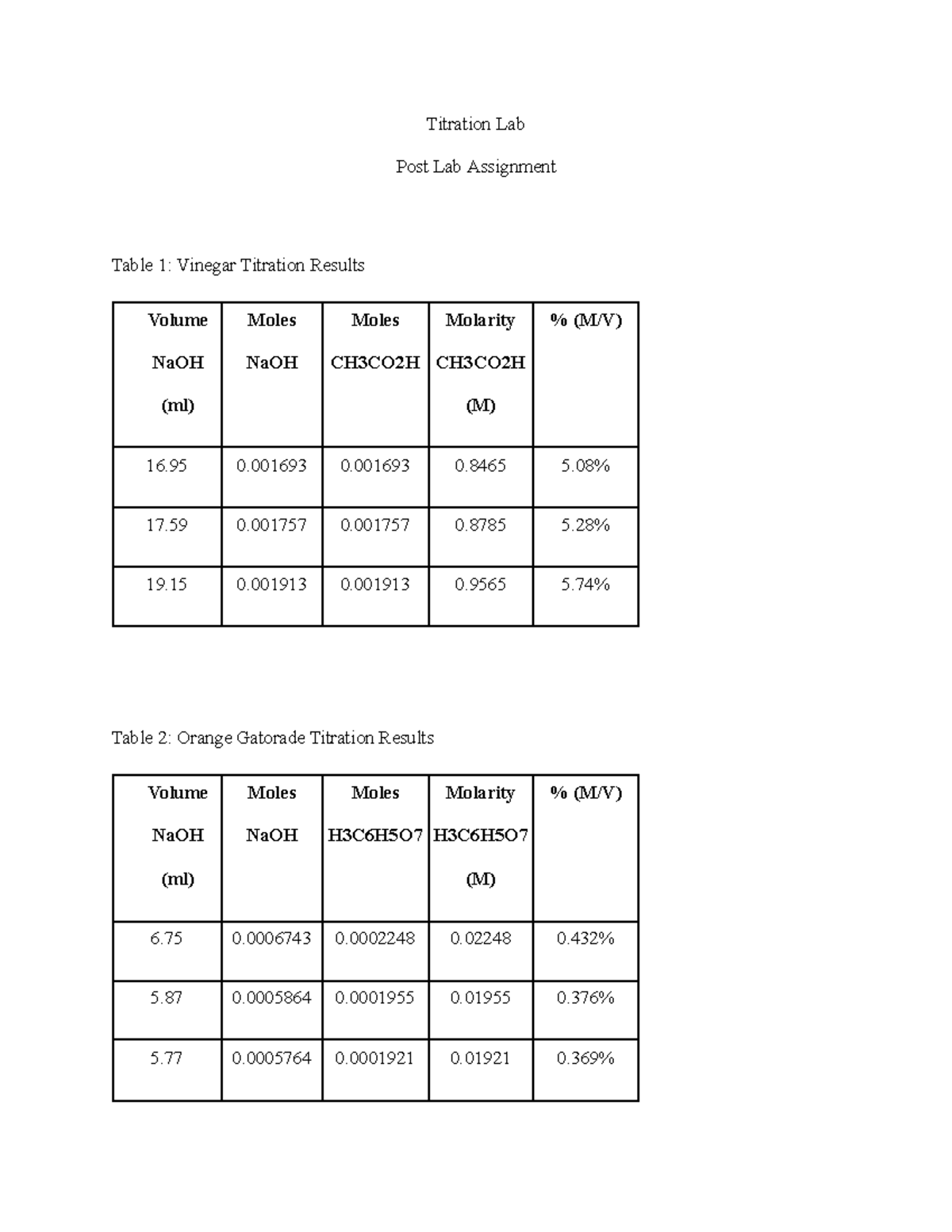
Titration Lab Titration Lab Post Lab Assignment Table 1 Vinegar Titration Results Volume NaOH
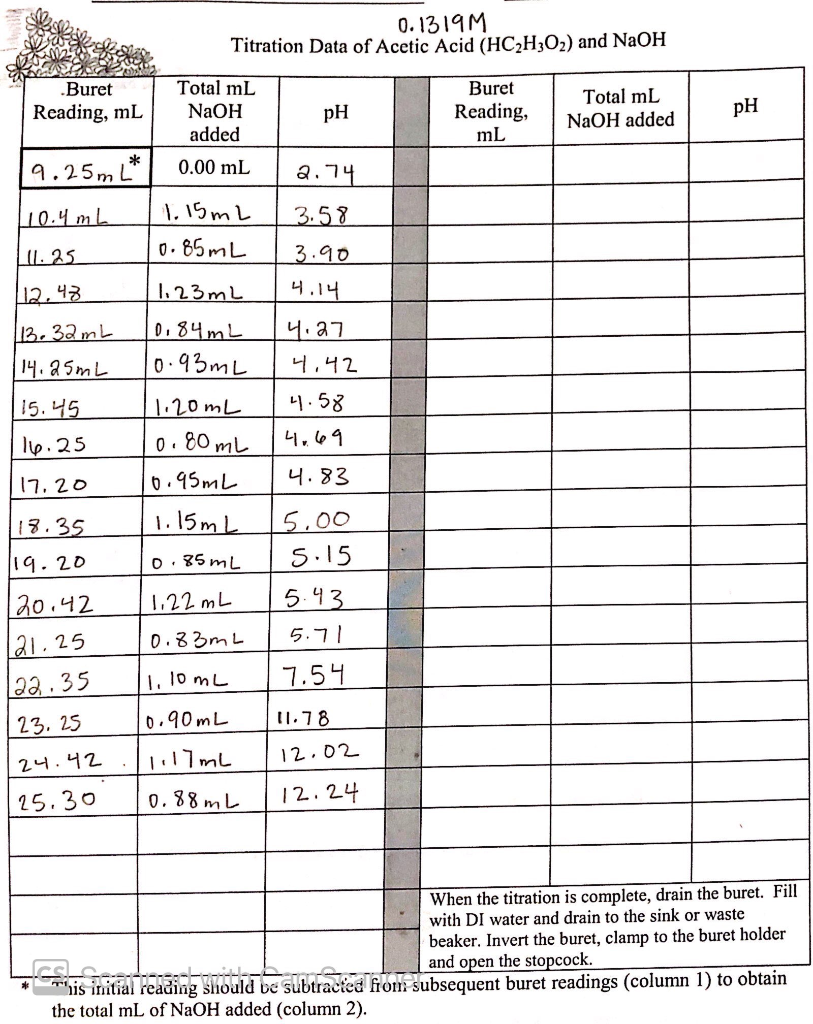
This is a titration table and we have 50.0ml 0.1319M

homework Determining iron content in spinach from titration Chemistry Stack Exchange

CDCP 02.28.11 Titration Excel Practice Example
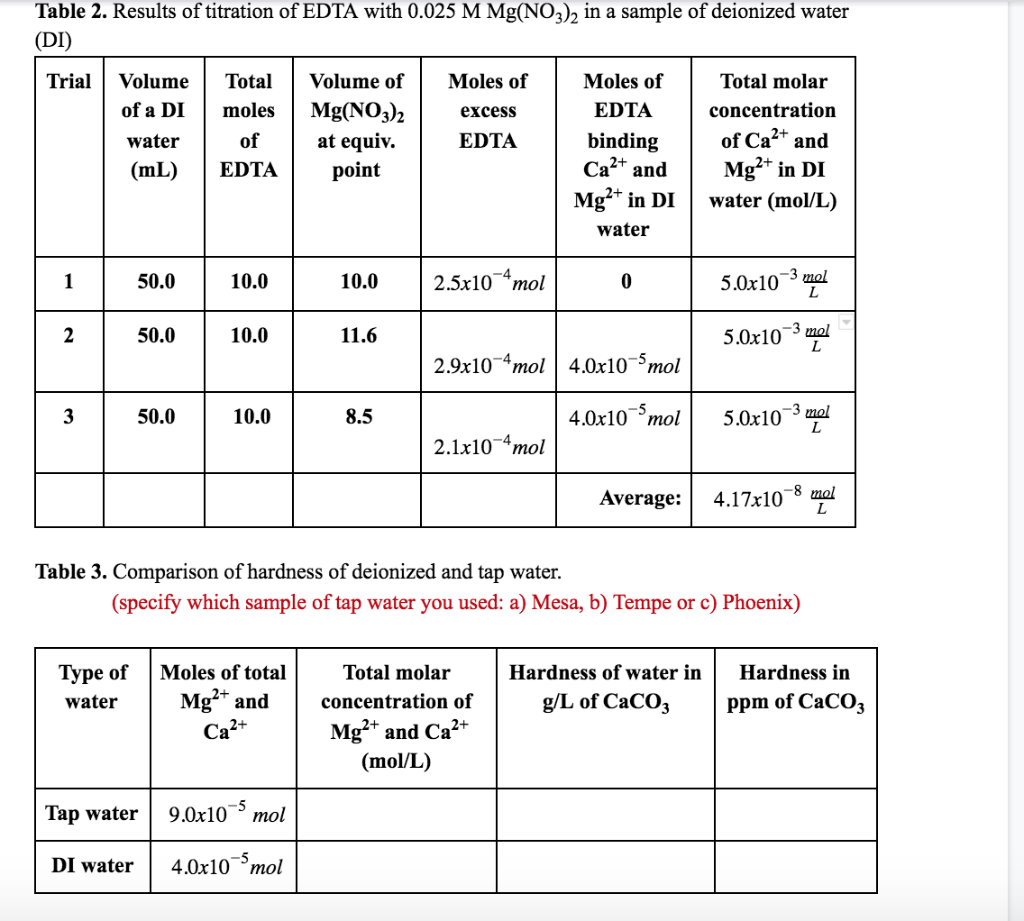
Table 2. Results of titration of EDTA with 0.025 M
Free photo Titration School, Medicine, Pharmaceutical Free Download Jooinn
[Solved] The table given shows data from the titration of four 10.0 mL… Course Hero

Titration Lab Report

Redox Titration Chemistry LibreTexts

Titration results of DEEA concentrations before and after the WWC… Download Table
Step 1: calculate the average titre without including the rough titration (28.50 mL). average titre = 26.75 + 26.70 +26.80 3 a v e r a g e t i t r e = 26.75 + 26.70 + 26.80 3. averagetitre = 26.75mL a v e r a g e t i t r e = 26.75 m L. Step 2: write a balanced chemical equation.. Equivalence point: point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. At the equivalence point in an acid-base titration, moles of base = moles of acid and the solution only contains salt and water. Diagram of equivalence point. Acid-base titrations are monitored by the change.